In this note we discuss isothermal, adiabatic, isobaric, and isochoric processes with an ideal gas. We begin by recalling a few basic principles from thermodynamics, and their application to a container of ideal gas, as shown in the figure below.
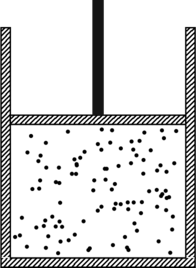
When the gas is at an absolute temperature ![]() , the molecules of the gas move around inside the container and bounce off its walls. Each gas molecule suffers a change in momentum upon such a collision, and the totality of all those collisions per unit time exerts a force on the walls of the container. …
, the molecules of the gas move around inside the container and bounce off its walls. Each gas molecule suffers a change in momentum upon such a collision, and the totality of all those collisions per unit time exerts a force on the walls of the container. …