JEE Advanced 2019 Paper 1, Question 9
One mole of a monatomic ideal gas goes through a thermodynamic cycle, as shown in the volume vs. temperature (![]() ) diagram. The correct statement(s) is/are:
) diagram. The correct statement(s) is/are:
[![]() is the gas constant]
is the gas constant]
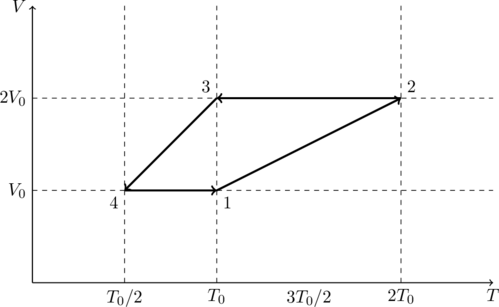
- Work done in this thermodynamic cycle (
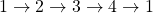 ) is
) is 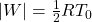 .
. - The above thermodynamic cycle exhibits only isochoric and adiabatic processes.
- The ratio of heat transfer during processes
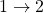 and
and 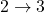 is
is 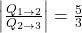 .
. - The ratio of heat transfer during processes
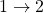 and
and 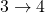 is
is 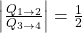 .
.
Related article: Thermodynamic processes on an ideal gas
Solution
An ideal gas obeys the relations
(1) ![]()
(2) ![]()
(3) ![]()
We …